Summary
■ IPG7236 is a first-in-class, orally administered, small-molecule antagonist of the chemokine receptor CCR8, establishing it as the global leader in this novel class of cancer immunotherapies.
■ IPG7236 is uniquely positioned with a dual-application strategy, targeting immunosuppressive regulatory T cells (Tregs) in oncology and pathogenic plasmacytoid dendritic cells (pDCs) in the autoimmune condition IgG4-Related Disease (IgG4-RD).
■ Developed with a novel chemical structure possessing superior druggability and high selectivity, IPG7236 has demonstrated a significant safety advantage over competing antibody-based approaches in clinical trials.
■ As the most advanced oral CCR8 antagonist in development, IPG7236 is poised to unlock the immune system's potential against solid tumors and provide a transformative new therapy for a rare disease with high unmet need.
IPG7236 in IgG4-RD
Mechanism of Action
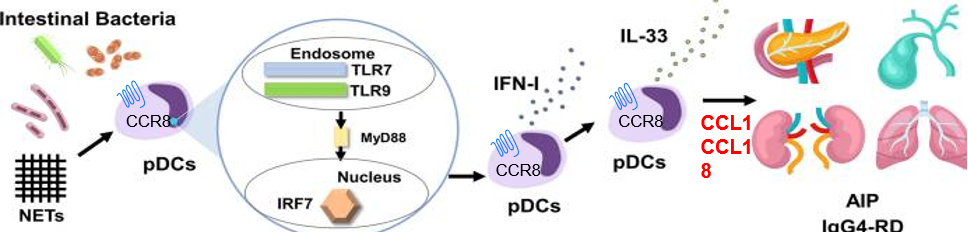
■ In IgG4-RD, CCR8 is predominantly expressed on pathogenic plasmacytoid dendritic cells (pDCs). These pDCs migrate to affected organs and produce inflammatory signals (IFN-I, IL-33) that drive the Th2 immune response, fibrosis, and production of pathogenic IgG4 antibodies.
■ IPG7236 is designed to block this CCR8-mediated pDC migration, thereby reducing their accumulation at lesion sites. This action breaks the chronic inflammatory cycle, alleviates inflammation and fibrosis, and helps restore normal immune tolerance.
Key Differentiation
■ Acts at the top of the pathogenic cascade: Given pDCs as the principal source of type-I IFN and IL-33, blocking pDC chemotaxis or depleting pathological pDCs could block the process before the B-cell and fibroblast amplification loops, whereas glucocorticoids, DMARDs and rituximab intervene only after these loops are established.
■ Disease-modifying potential instead of broad immunosuppression: Current treatments such as glucocorticoids, DMARDs and Rituximab are constrained by their limited efficacy and inevitable safety concerns. In contrast, CCR8 antagonists have shown promising efficacy and excellent safety in experimental AIP/IgG4-RD, thereby providing novel therapeutic approaches to IgG4-RD.
■ Vast Untapped Market Potential: IgG4-RD represents an orphan disease with a growing patient population and a clear need for safer, more effective treatments.
Current Development & Status
■ Phase 1 (Healthy Subjects): An initial Phase 1 trial in healthy subjects in Australia demonstrated that IPG7236 is well-tolerated, with linear pharmacokinetics supporting its development.
■ Strong Safety Foundation: The favorable safety and tolerability profile demonstrated in the ongoing oncology trials provides a strong foundation for its development in the IgG4-RD patient population.
■ Next Steps: A Phase 2 clinical trial for IgG4-RD is under preparation.
In vivo Properties
Using a surrogate anti-CCR8 antibody (IPG0521) in a mouse model of IgG4-RD, treatment resulted in significant, dose-dependent therapeutic effects:
> In a mouse model of IgG4-RD, treatment with IPG0521 resulted in significant and dose-dependent therapeutic effects.
> Reduced Organ Inflammation: Markedly reduced inflammation in multiple organs such as pancreatic tissue, salivary tissue, etc, which is characterized by decreased immune cell infiltration and parenchymal destruction. Significantly attenuated pancreatic fibrosis in the affected organs in a dose-dependent manner.
> Downregulated Key Cytokines: Significantly downregulated the expression of key pro-inflammatory and pro-fibrotic cytokines (IL-6, IL-1β, IFN-γ, IL-10, and TGF-β) in affected organs.
IPG7236 in Oncology
Mechanism of Action

■ CCR8 is predominantly expressed on tumor-infiltrating Tregs, which are a primary driver of immunosuppression within the tumor microenvironment. These Tregs prevent the body’s natural antitumor immune response.
■ IPG7236 works by selectively blocking the CCL1-CCR8 signaling pathway, which disrupts the recruitment of these immunosuppressive Tregs to the tumor site.
■ This alleviates the immunosuppressive shield, enhancing the infiltration and activity of tumor-killing CD8+ T cells and enabling the immune system to effectively recognize and attack cancer cells.
Key Differentiation
■ Oral Small-Molecule Advantage: IPG7236's oral administration provides significant advantages over large-molecule antibody competitors in terms of manufacturing cost, distribution, and patient compliance.
■ Novel Mechanism of Action (Signaling Blockade vs. Cell Depletion): Unlike competing ADCC-enhanced antibodies that deplete all CCR8-expressing cells (including healthy ones), IPG7236 selectively blocks Treg function without causing widespread cell death. This targeted mechanism is anticipated to deliver a significantly superior safety profile by avoiding on-target, off-tumor toxicity.
■ Superior Safety Profile: In the ongoing Phase 1/2a clinical trial, IPG7236 has been escalated up to 1000 mg BID without any drug-related Serious Adverse Events (SAEs) observed, highlighting its excellent tolerability.
■ Synergy with PD-1 Inhibitors: Pre-clinical data demonstrates a strong synergistic effect with anti-PD-1 antibodies, positioning IPG7236 as a promising therapy to overcome resistance to current checkpoint inhibitors.
Current Development & Status
■ Phase 1/2a MRCT (Ongoing): A multi-center clinical trial for advanced solid tumors is ongoing in the U.S. and China.
■ Next Steps: A Cinical trial is planned to commerce to evaluate IPG7236 in combination with an anti-PD-1 therapy for patients with solid tumor.
In vivo Properties
■ Tumor Microenvironment Modulation: In a humanized breast cancer model, IPG7236 treatment caused a significant, dose-dependent decrease in tumor-infiltrating Tregs and a corresponding dose-dependent increase in tumor-killing CD8+ T cells.

■ Monotherapy Antitumor Activity: IPG7236 demonstrated potent, dose-dependent inhibition of tumor growth as a monotherapy in the same humanized mouse model.
■ Synergistic Efficacy with Anti-PD-1: In a combination study, IPG7236 (TGI of 47.2%) together with a PD-1 antibody resulted in a significantly enhanced anti-cancer effect (TGI of 73.8%), while the PD-1 antibody alone showed no significant effect.

Publications
•Wu Y, Xi J, Li Y, Li Z, Zhang Y, Wang J, Fan GH. Discovery of a Potent and Selective CCR8 Small Molecular Antagonist IPG7236 for the Treatment of Cancer. J Med Chem. 2023 Apr 13;66(7):4548-4564. doi: 10.1021/acs.jmedchem.3c00030. Epub 2023 Mar 29. PMID: 36988587.
Copyright © 2025 Nanjing Immunophage Biomedical Co.,Ltd
All Rights Reserved. 苏ICP备2022026466号-1  苏公网安备 32011202000830号
苏公网安备 32011202000830号
